What is wrong with my wine?
Brought to you by Carl DiManno of Winesecrets East [email protected]
Wine Flaw Identification, Prevention and Mitigation
Carl DiManno
At some point, every winemaker will experience a flaw in their wine. Despite their best efforts to monitor and maintain their wine, a small portion of it will develop aromas or flavors that are not commercially acceptable. While blending can be a viable solution, oftentimes it results in a greater volume of tainted wine.
Like many issues, prevention is the best solution to flawed wine. However, in the event of an issue, the ability to identify the issue and take corrective action is one of the most important aspects of modern-day winemaking. The following discusses common wine flaws, their typical identifying traits, prevention steps and techniques for eliminating the issue in finished wine.
Sulfur Like Odors
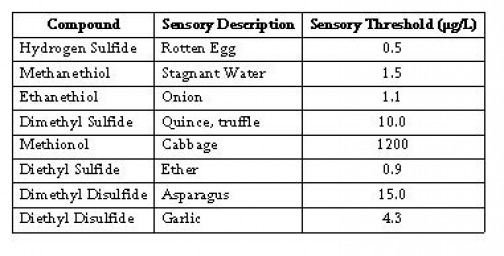
Sulfur Like Odors (SLO) are common in wine. They range from rotten egg aroma to other unappealing characters such as onion, garlic, cabbage and burnt rubber. All wines contain sulfur compounds, though most are below the threshold level for human detection. However, depending on the winemaking technique or the condition of the wine, SLO may become prevalent and impact the sensory properties of a wine.
Table 1 summarizes typical SLOs, their aromas and their detection levels. The quantities of SLO needed for threshold detection level is small, in the parts per billion range (µg/L). The table display threshold levels in a model wine. Actual detectable level is any wine will depend on the composition of that particular wine, including phenolic content, volatile aroma components and polysaccharide and protein concentrations.
Beyond their impact on aroma, SLOs can negatively impact wine mouthfeel. Excessive SLOs can contribute a mineral or bitter taste to the wine. Sulfur components can also add astringency to a wine.
Table 1: Common Sulfur Like Odors in Wine
The bulk of sulfur compounds in wine are formed during fermentation. While all fermentations result in some sulfur compounds, yeast that are starved for nitrogen will produce significantly more sulfur-bearing molecules. During the process of converting sugar to alcohol, yeast require some nitrogen to reproduce. Once the available nitrogen in a must or juice is exhausted, the yeast will break down amino acids, including sulfur-containing amino acids cysteine and methionine. The byproduct of this breakdown is hydrogen sulfide (H2S), which smells like rotten eggs.
During fermentation, much of the H2S will “blow-off” or leave with the high gas volume of CO2 generated by the yeast. If, however, all the H2S is not removed, the wine will retain the off-odor and higher sulfides (thiols, disulfides) may develop, which are harder to remove from wine.
To minimize H2S formation during fermentation, proper must/juice nitrogen levels are required. Grapevines do not produce fruit to feed yeast. As such, grapes are not optimized for fermentations. Supplemental nitrogen is needed for clean ferments. Excess nitrogen will create its own issues, including extremely fast fermentation and loss of varietal character. An optimal level of nitrogen is required.
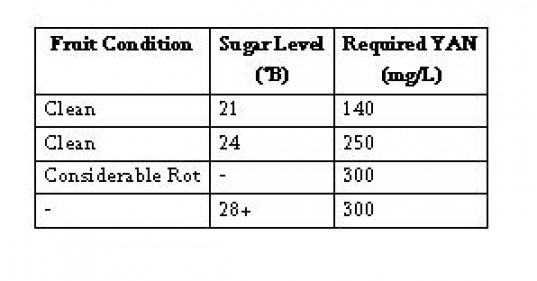
Measure the amount of ammonia (NH3) and primary amino nitrogen (PAN). Many labs offer one day turnaround for this analysis during harvest. In house, a formol titration or enzymatic tests are available to determine yeast assimilable nitrogen (YAN). YAN is equal to NH3 + PAN. Depending on the fruit condition, differing levels of YAN are required (Table 2).
The bulk of sulfur compounds in wine are formed during fermentation. While all fermentations result in some sulfur compounds, yeast that are starved for nitrogen will produce significantly more sulfur-bearing molecules. During the process of converting sugar to alcohol, yeast require some nitrogen to reproduce. Once the available nitrogen in a must or juice is exhausted, the yeast will break down amino acids, including sulfur-containing amino acids cysteine and methionine. The byproduct of this breakdown is hydrogen sulfide (H2S), which smells like rotten eggs.
During fermentation, much of the H2S will “blow-off” or leave with the high gas volume of CO2 generated by the yeast. If, however, all the H2S is not removed, the wine will retain the off-odor and higher sulfides (thiols, disulfides) may develop, which are harder to remove from wine.
To minimize H2S formation during fermentation, proper must/juice nitrogen levels are required. Grapevines do not produce fruit to feed yeast. As such, grapes are not optimized for fermentations. Supplemental nitrogen is needed for clean ferments. Excess nitrogen will create its own issues, including extremely fast fermentation and loss of varietal character. An optimal level of nitrogen is required.
Measure the amount of ammonia (NH3) and primary amino nitrogen (PAN). Many labs offer one day turnaround for this analysis during harvest. In house, a formol titration or enzymatic tests are available to determine yeast assimilable nitrogen (YAN). YAN is equal to NH3 + PAN. Depending on the fruit condition, differing levels of YAN are required (Table 2).
Table 2: Required YAN Levels
YAN is available from proprietary supplements including GoFerm (Thiazote, Nutristart) and Superfood. Diammonium Phosphate (DAP) is another supplement. DAP only provides NH3 to the ferment. Consider using the other products in conjunction with DAP for a more balanced fermentation environment, including primary amino nitrogen and vitamins.
Proper must nutrition will reduce the amount of sulfur compounds in a wine and decrease the chance of experiencing SLO. A wine with a healthy fermentation may develop SLO under reduced conditions, however.
Aging wines will not benefit from large amounts of oxygen. They do, however, require some oxygen for aging. Storing wines with very little or no oxygen can result in a reductive environment. Under these conditions, trace amounts of sulfur compounds can form species with very low threshold levels, resulting in SLO.
If a wine is displaying the symptoms of SLO, the first approach to correction is aeration. Providing gross oxygenation through a splash rack or other method can liberate the volatile H2S from the wine, reducing its levels below threshold. The oxygen can reverse the reductive environment, converting the low threshold thiol compounds to higher sensory threshold disulfide compounds.
In the event that aeration does not work or the levels of sulfur compounds are sufficiently high that disulfides are detectable, copper is a viable option. The winemaker should treat the wines with ascorbic acid (50 mg/L) and SO2 prior to copper treatment. This forces a reaction that converts the untreatable disulfides into treatable thiols. The copper binds with the sulfur and precipitates out of solution. Wines treated with copper should be racked before further processing.
Copper is the last line of defense for SLO. There are significant disadvantages to copper treatment. By law, a wine may only contain 0.5 mg/L copper. Copper fining can strip a wine of volatile characteristics leaving it flat and uninteresting.
Volatile Acidity
All wines contain volatile acidity and it contributes to the overall acidity and aroma of wines. Acids are considered volatile when they are steam distillable and include acetic, butyric, formic, propionic acids and ethyl acetate. Excessive volatile acidity (VA), typically acetic acid, can cause a wine to take on aromas of vinegar, salad dressing, ketchup and barbeque sauce while reducing varietal character. VA is detectable in the 0.6 – 0.9 g/L level. Legal limits for VA are 1.4 g/L in red wine and 1.2 g/L in white wine.
Some acetic acid is formed during fermentation but the bulk of problematic volatile acidity occurs during storage and malolactic fermentation. The Acetobacter bacteria consumes ethanol in the presence of oxygen and forms acetic acid. Likewise, lactobacillus consumes residual sugar in stored wine, creating acetic acid.
Ethyl acetate can also form as acetic acid is produced. Ethyl acetate (EtAc) has a distinct nail polish remover characteristic. EtAc also forms early in the winemaking process, as spoilage yeast that come in from the vineyards can form ethyl acetate as a by-product.
To prevent EtAc, musts and juice should be treated with SO2 if allowed to cold soak or cold settle. Likewise, cold soak/settle should be cold (45°F) to prevent the establishment of spoilage yeast prior to cultured yeast inoculation. If there is a question about maintaining cold temperatures prior to fermentation, inoculate the must at the destemmer with the appropriate yeast. In the event cooling is lost, the fermentation will initiate with the proper yeast.
Preventing VA is stored wine involves sound winemaking practices. Acetobacter requires oxygen to ferment. Topping barrels and gassing tanks is the best way to prevent VA production. SO2 is also critical to suppressing the establishment of VA producing bacteria. In the case of lactobacillus, wines with residual sugar should be sterile filtered or treated with a sterilant such as Velcorin prior to storage.
Once VA is established, it is hard to remove. The first step is to filter the wine to remove the VA causing bacteria and stabilize the acetic acid level. Once no more acetic acid is being produced, the issue can be addressed.
Blending is an option. However, as a typical wine contains 0.4 g/L VA, a considerable amount of “clean” wine is needed to blend a wine with a high VA down below the sensory threshold of 0.6 – 0.9 g/L. Also, if the wine is not completely free of the acetic acid bacteria, there is a risk of inoculating a larger batch of wine with the VA causing microbe.
Some winemakers swear by lees fining. Oxygenated wine lees contain proteins and polysaccharides. They could potentially bind with the acetic acid rendering it non-volatile. Clean lees are needed. Utilization of lees stored under less than ideal conditions might just lead to adding more bacteria to the recently filtered wine.
One proven method for the removal of acetic acid in wine is reverse osmosis. Reverse osmosis (RO) is a high pressure micro filter that can split water, alcohol and acetic acid out of a wine, treat the acid with an ion exchange resin and return the wine without the acetic acid taint. In the filter, the smallest molecular weight components (water, alcohol and acetic acid) penetrate the filter membrane (permeate) while the remaining wine does not penetrate the membrane (retentate). Only the permeate is treated, leaving the retentate intact and minimizing impact on the wine.
Winesecrets offers mobile reverse osmosis for the treatment of VA. Once the level of VA is determined, Winesecrets will provide a quotation for VA removal. The RO and a professional technician will arrive at the winery and treat the wine in questions. Contact Carl DiManno at 703.728.7977 or [email protected] to discuss volatile acidity reduction through reverse osmosis.
Brettanomyces
Brettanomyces bruxellensis is a spoilage yeast responsible for off-putting aromas in wine. Wines infected with Brettanomyces (Brett) often display aromas of band-aid, antiseptic, barnyard, horse blanket, wet cardboard and wet dog. The flavor of the wine is often affected by a metallic taste.
In some wines and some wine regions, a little bit for Brett can be consider the “house style.” Many of the reds of Bordeaux exhibit these characteristics. The contamination by Brett is a matter of degree. These aromas in relatively large quantities can overwhelm a wine and make it undrinkable.
Brett typically occurs in red wines stored in barrels. Warm conditions and low SO2 are ideal conditions for Brett. Unfortunately, these conditions are also ideal for promoting Malolactic fermentation. High pH and residual sugar also promote Brett growth.
The verification of Brett presence can be verified by laboratory culture or the detection of the chemical compounds 4-ethylphenol and 4-ethylguaiacol (4EP/4EG). Brettanomyces produces a myriad of aroma compounds. 4EP/4EG serve are markers for the presence of Brett. Where there is 4EP/4EG, there is Brett.
Brett comes in from the vineyard. It does not compete well with other wine yeasts and will not ferment juice. However, once fermentation is complete and there is no competition, Brett can become established. Barrels are ideal environments for Brett survival and wines are typically inoculated by old barrels as Brett has gotten established in empty barrels that previously held wine.
Preventing Brett is a matter of cellar sanitation. Cool cellars and appropriate levels of SO2 go a long way to preventing Brett formation. Wines with higher pH promote Brett growth and require higher levels of SO2 to gain the appropriate killing power. Brett easily feeds off of residual sugar. Wines with any residual sugar or high pH require special vigilance in the cellar to prevent Brett formation.
Winesecrets offers a reverse osmosis process for the removal of “Bretty” aromas. Once the wine has been filtered to remove the spoilage yeast, Winesecrets can process it with a reverse osmosis membrane with a slightly larger pore size than that used for VA. The larger pore allows molecules as large as 4EP/4EG (MW 152) to pass through the membrane. This permeate is treated with a carbon block filter, removing 4EP/4EG and other similarly sized spoilage compounds. Contact Carl DiManno at 703.728.7977 or [email protected] to discuss Brett taint reduction through reverse osmosis.
Pyrazine
Pyrazines are aroma compounds found in many green vegetables, including bell peppers, chilies and peas. Pyrazines occur in grapes as well. At veraison, the Pyrazine level in grapes is at its peak. As ripening occurs, the Pyrazine level diminishes. As such, fruit that is underripe, shaded or ripens unevenly may contain elevated levels of Pyrazine.
Sauvignons (Cabernet, Blanc) have higher levels of pyrazines than other varieties. In a Sauvignon Blanc, grassy and green notes are expected. In a red, they can be off-putting. Pyrazines are detectable in very small concentrations. In whites, the “green” aromas can be detected at 2 ng/L. In reds, Pyrazine can be detected at 10 ng/L (parts per trillion). By way of example, 7 ml of Pyrazine poured into an ocean-going supertanker full of red wine could be detected.
Prevention of excessive pyrazines starts in the vineyard. Ripe fruit that has received adequate sunlight will be low in Pyrazine. Canopy management to prevent shading of cluster and other leaves will promote even ripening and minimize “green” components. Balancing fruit load will also promote ripening. To prevent over cropping and reduced ripeness, consider dropping fruit.
Winemaking practices can reduce Pyrazine extraction. 53% of grape pyrazines are in the cluster stems when 31% is in the seeds. Minimize stem contact by destemming quickly and limiting stem pieces in the fermenter. Drop seeds (délestage) from the fermenter if there is a real concern of an overabundance of Pyrazine.
Elimination of Pyrazine is difficult and there is no proven technological methods at this time. Blending is an option along with masking the aroma with oak or other odorants.
Cork Taint
2,4,6-trichloroanisole (TCA) is a compound that cause a wine to exhibit a musty or wet cellar aroma. Like Pyrazine, it’s extremely potent and can be detected in white wines at 2 parts per trillion and red wines at 5 parts per trillion.
TCA is produced by a mold. The mold converts chlorine and chlorophenols into TCA. In the past, corks were often treated with bleach. Left wet, the mold would become established and consume the chlorine in the bleach, forming TCA. Cork manufacturers have made great strides in preventing TCA contamination. It is still a risk, however. Pressure treated wood contains chlorophenols and are hard to keep dry and clean. This is an ideal environment for the production of TCA.
Mitigation steps for a cellar contaminated with TCA usually involve burning it to the ground. It is exceedingly difficult to remove once established. Prevention is of the utmost importance. All chlorine cleaning products should be eliminated from the cellar. Exposed wood, especially pressure treated wood should be kept dry and wine kept out of contact with it. Barrels should be sanitized and treated with ozone if possible.
Due to its extremely small sensory threshold, blending a wine with TCA is typically not an option. Some wineries have reported success with TCA reduction by running wines over Saran wrap. A large surface area of Saran wrap in contact with a small volume of wine can bind the TCA and reduce its sensory impact.
Oxidation and Maderization
While traces of oxygen are needed to properly age a wine, gross oxygenation will damage a wine. Both red and white wines will appear brown once oxidized. The fruit aromas are muted and the wine may have attributes of bruised apple or may have an accompanying volatile acidity issue. Barrels that are not regularly topped or tanks not regularly gassed will typically lead to oxidation.
Maderization is a similar condition. Literally meaning cooked, the wine will take on the characteristics of sherry. One primary source for this is barrels that have not been topped and have had a yeast film grow on the wine surface. The candida film is the same microbe that is used in sherry production. However, these attributes are not palatable in a table wine.
Prevention is relatively simple. Keep oxygen away from wine. Top barrels and gas tanks. In barrels, stir lees as the autolyzed yeast have antioxidant properties. Maintain SO2 levels. SO2 acts as an oxygen scavenger.
Winesecrets offers Ultrafiltration for the removal of brown color or maderized characters. Ultrafiltration uses a membrane to selectively allow low molecular weight components to pass through the membrane, leaving large browned tannins in the retentate. The resultant wine will have a higher concentration of esters and unpolymerized color species and will exhibit much of the fruit that was masked by the browning components. . Contact Carl DiManno at 703.728.7977 or [email protected] to discuss Ultrafiltration for the oxidation and maderization characters of wine.
Other Taints
There are several species of lactic acid bacteria. All can contribute to converting malic acid to lactic acid in malolactic fermentation (MLF). Some, however will provide unwanted byproducts. While the Oenicoccus oeni lactic acid bacteria (LAB) imparts desirable characteristics, other LAB responsible for fermenting sauerkraut and sausage do not. Other undesireable characters that can result from LAB include diacetyl (movie theater popcorn butter, rancid butter) and butyric acid (spoiled cheese, sweat).
Prevention of these LAB off-characters can typically be handled by inoculating for MLF with a cultured bacteria along with its nutritional supplement. Mitigation is difficult. Aeration can “blow-off” some of the offending aromas. Suppression is important to stop further production of the off-aroma. SO2 and limiting oxygen will stop the microbes so that the wine can be filtered and the off-aroma blended away. In the case of diacetyl, it is often prudent to wait, especially if MLF is ongoing. Formation of diacetyl is part of the MLF pathway. It may dissipate once MLF is complete.
Excessive sulfur dioxide (SO2) can impart a matchstick aroma to wine. High SO2 additions or the lowering of wine pH can result in excessive SO2 aroma. Aeration or oxygenation will reduce this character as the SO2 will bind with free oxygen and reduce the volatile portion of the component.
YAN is available from proprietary supplements including GoFerm (Thiazote, Nutristart) and Superfood. Diammonium Phosphate (DAP) is another supplement. DAP only provides NH3 to the ferment. Consider using the other products in conjunction with DAP for a more balanced fermentation environment, including primary amino nitrogen and vitamins.
Proper must nutrition will reduce the amount of sulfur compounds in a wine and decrease the chance of experiencing SLO. A wine with a healthy fermentation may develop SLO under reduced conditions, however.
Aging wines will not benefit from large amounts of oxygen. They do, however, require some oxygen for aging. Storing wines with very little or no oxygen can result in a reductive environment. Under these conditions, trace amounts of sulfur compounds can form species with very low threshold levels, resulting in SLO.
If a wine is displaying the symptoms of SLO, the first approach to correction is aeration. Providing gross oxygenation through a splash rack or other method can liberate the volatile H2S from the wine, reducing its levels below threshold. The oxygen can reverse the reductive environment, converting the low threshold thiol compounds to higher sensory threshold disulfide compounds.
In the event that aeration does not work or the levels of sulfur compounds are sufficiently high that disulfides are detectable, copper is a viable option. The winemaker should treat the wines with ascorbic acid (50 mg/L) and SO2 prior to copper treatment. This forces a reaction that converts the untreatable disulfides into treatable thiols. The copper binds with the sulfur and precipitates out of solution. Wines treated with copper should be racked before further processing.
Copper is the last line of defense for SLO. There are significant disadvantages to copper treatment. By law, a wine may only contain 0.5 mg/L copper. Copper fining can strip a wine of volatile characteristics leaving it flat and uninteresting.
Volatile Acidity
All wines contain volatile acidity and it contributes to the overall acidity and aroma of wines. Acids are considered volatile when they are steam distillable and include acetic, butyric, formic, propionic acids and ethyl acetate. Excessive volatile acidity (VA), typically acetic acid, can cause a wine to take on aromas of vinegar, salad dressing, ketchup and barbeque sauce while reducing varietal character. VA is detectable in the 0.6 – 0.9 g/L level. Legal limits for VA are 1.4 g/L in red wine and 1.2 g/L in white wine.
Some acetic acid is formed during fermentation but the bulk of problematic volatile acidity occurs during storage and malolactic fermentation. The Acetobacter bacteria consumes ethanol in the presence of oxygen and forms acetic acid. Likewise, lactobacillus consumes residual sugar in stored wine, creating acetic acid.
Ethyl acetate can also form as acetic acid is produced. Ethyl acetate (EtAc) has a distinct nail polish remover characteristic. EtAc also forms early in the winemaking process, as spoilage yeast that come in from the vineyards can form ethyl acetate as a by-product.
To prevent EtAc, musts and juice should be treated with SO2 if allowed to cold soak or cold settle. Likewise, cold soak/settle should be cold (45°F) to prevent the establishment of spoilage yeast prior to cultured yeast inoculation. If there is a question about maintaining cold temperatures prior to fermentation, inoculate the must at the destemmer with the appropriate yeast. In the event cooling is lost, the fermentation will initiate with the proper yeast.
Preventing VA is stored wine involves sound winemaking practices. Acetobacter requires oxygen to ferment. Topping barrels and gassing tanks is the best way to prevent VA production. SO2 is also critical to suppressing the establishment of VA producing bacteria. In the case of lactobacillus, wines with residual sugar should be sterile filtered or treated with a sterilant such as Velcorin prior to storage.
Once VA is established, it is hard to remove. The first step is to filter the wine to remove the VA causing bacteria and stabilize the acetic acid level. Once no more acetic acid is being produced, the issue can be addressed.
Blending is an option. However, as a typical wine contains 0.4 g/L VA, a considerable amount of “clean” wine is needed to blend a wine with a high VA down below the sensory threshold of 0.6 – 0.9 g/L. Also, if the wine is not completely free of the acetic acid bacteria, there is a risk of inoculating a larger batch of wine with the VA causing microbe.
Some winemakers swear by lees fining. Oxygenated wine lees contain proteins and polysaccharides. They could potentially bind with the acetic acid rendering it non-volatile. Clean lees are needed. Utilization of lees stored under less than ideal conditions might just lead to adding more bacteria to the recently filtered wine.
One proven method for the removal of acetic acid in wine is reverse osmosis. Reverse osmosis (RO) is a high pressure micro filter that can split water, alcohol and acetic acid out of a wine, treat the acid with an ion exchange resin and return the wine without the acetic acid taint. In the filter, the smallest molecular weight components (water, alcohol and acetic acid) penetrate the filter membrane (permeate) while the remaining wine does not penetrate the membrane (retentate). Only the permeate is treated, leaving the retentate intact and minimizing impact on the wine.
Winesecrets offers mobile reverse osmosis for the treatment of VA. Once the level of VA is determined, Winesecrets will provide a quotation for VA removal. The RO and a professional technician will arrive at the winery and treat the wine in questions. Contact Carl DiManno at 703.728.7977 or [email protected] to discuss volatile acidity reduction through reverse osmosis.
Brettanomyces
Brettanomyces bruxellensis is a spoilage yeast responsible for off-putting aromas in wine. Wines infected with Brettanomyces (Brett) often display aromas of band-aid, antiseptic, barnyard, horse blanket, wet cardboard and wet dog. The flavor of the wine is often affected by a metallic taste.
In some wines and some wine regions, a little bit for Brett can be consider the “house style.” Many of the reds of Bordeaux exhibit these characteristics. The contamination by Brett is a matter of degree. These aromas in relatively large quantities can overwhelm a wine and make it undrinkable.
Brett typically occurs in red wines stored in barrels. Warm conditions and low SO2 are ideal conditions for Brett. Unfortunately, these conditions are also ideal for promoting Malolactic fermentation. High pH and residual sugar also promote Brett growth.
The verification of Brett presence can be verified by laboratory culture or the detection of the chemical compounds 4-ethylphenol and 4-ethylguaiacol (4EP/4EG). Brettanomyces produces a myriad of aroma compounds. 4EP/4EG serve are markers for the presence of Brett. Where there is 4EP/4EG, there is Brett.
Brett comes in from the vineyard. It does not compete well with other wine yeasts and will not ferment juice. However, once fermentation is complete and there is no competition, Brett can become established. Barrels are ideal environments for Brett survival and wines are typically inoculated by old barrels as Brett has gotten established in empty barrels that previously held wine.
Preventing Brett is a matter of cellar sanitation. Cool cellars and appropriate levels of SO2 go a long way to preventing Brett formation. Wines with higher pH promote Brett growth and require higher levels of SO2 to gain the appropriate killing power. Brett easily feeds off of residual sugar. Wines with any residual sugar or high pH require special vigilance in the cellar to prevent Brett formation.
Winesecrets offers a reverse osmosis process for the removal of “Bretty” aromas. Once the wine has been filtered to remove the spoilage yeast, Winesecrets can process it with a reverse osmosis membrane with a slightly larger pore size than that used for VA. The larger pore allows molecules as large as 4EP/4EG (MW 152) to pass through the membrane. This permeate is treated with a carbon block filter, removing 4EP/4EG and other similarly sized spoilage compounds. Contact Carl DiManno at 703.728.7977 or [email protected] to discuss Brett taint reduction through reverse osmosis.
Pyrazine
Pyrazines are aroma compounds found in many green vegetables, including bell peppers, chilies and peas. Pyrazines occur in grapes as well. At veraison, the Pyrazine level in grapes is at its peak. As ripening occurs, the Pyrazine level diminishes. As such, fruit that is underripe, shaded or ripens unevenly may contain elevated levels of Pyrazine.
Sauvignons (Cabernet, Blanc) have higher levels of pyrazines than other varieties. In a Sauvignon Blanc, grassy and green notes are expected. In a red, they can be off-putting. Pyrazines are detectable in very small concentrations. In whites, the “green” aromas can be detected at 2 ng/L. In reds, Pyrazine can be detected at 10 ng/L (parts per trillion). By way of example, 7 ml of Pyrazine poured into an ocean-going supertanker full of red wine could be detected.
Prevention of excessive pyrazines starts in the vineyard. Ripe fruit that has received adequate sunlight will be low in Pyrazine. Canopy management to prevent shading of cluster and other leaves will promote even ripening and minimize “green” components. Balancing fruit load will also promote ripening. To prevent over cropping and reduced ripeness, consider dropping fruit.
Winemaking practices can reduce Pyrazine extraction. 53% of grape pyrazines are in the cluster stems when 31% is in the seeds. Minimize stem contact by destemming quickly and limiting stem pieces in the fermenter. Drop seeds (délestage) from the fermenter if there is a real concern of an overabundance of Pyrazine.
Elimination of Pyrazine is difficult and there is no proven technological methods at this time. Blending is an option along with masking the aroma with oak or other odorants.
Cork Taint
2,4,6-trichloroanisole (TCA) is a compound that cause a wine to exhibit a musty or wet cellar aroma. Like Pyrazine, it’s extremely potent and can be detected in white wines at 2 parts per trillion and red wines at 5 parts per trillion.
TCA is produced by a mold. The mold converts chlorine and chlorophenols into TCA. In the past, corks were often treated with bleach. Left wet, the mold would become established and consume the chlorine in the bleach, forming TCA. Cork manufacturers have made great strides in preventing TCA contamination. It is still a risk, however. Pressure treated wood contains chlorophenols and are hard to keep dry and clean. This is an ideal environment for the production of TCA.
Mitigation steps for a cellar contaminated with TCA usually involve burning it to the ground. It is exceedingly difficult to remove once established. Prevention is of the utmost importance. All chlorine cleaning products should be eliminated from the cellar. Exposed wood, especially pressure treated wood should be kept dry and wine kept out of contact with it. Barrels should be sanitized and treated with ozone if possible.
Due to its extremely small sensory threshold, blending a wine with TCA is typically not an option. Some wineries have reported success with TCA reduction by running wines over Saran wrap. A large surface area of Saran wrap in contact with a small volume of wine can bind the TCA and reduce its sensory impact.
Oxidation and Maderization
While traces of oxygen are needed to properly age a wine, gross oxygenation will damage a wine. Both red and white wines will appear brown once oxidized. The fruit aromas are muted and the wine may have attributes of bruised apple or may have an accompanying volatile acidity issue. Barrels that are not regularly topped or tanks not regularly gassed will typically lead to oxidation.
Maderization is a similar condition. Literally meaning cooked, the wine will take on the characteristics of sherry. One primary source for this is barrels that have not been topped and have had a yeast film grow on the wine surface. The candida film is the same microbe that is used in sherry production. However, these attributes are not palatable in a table wine.
Prevention is relatively simple. Keep oxygen away from wine. Top barrels and gas tanks. In barrels, stir lees as the autolyzed yeast have antioxidant properties. Maintain SO2 levels. SO2 acts as an oxygen scavenger.
Winesecrets offers Ultrafiltration for the removal of brown color or maderized characters. Ultrafiltration uses a membrane to selectively allow low molecular weight components to pass through the membrane, leaving large browned tannins in the retentate. The resultant wine will have a higher concentration of esters and unpolymerized color species and will exhibit much of the fruit that was masked by the browning components. . Contact Carl DiManno at 703.728.7977 or [email protected] to discuss Ultrafiltration for the oxidation and maderization characters of wine.
Other Taints
There are several species of lactic acid bacteria. All can contribute to converting malic acid to lactic acid in malolactic fermentation (MLF). Some, however will provide unwanted byproducts. While the Oenicoccus oeni lactic acid bacteria (LAB) imparts desirable characteristics, other LAB responsible for fermenting sauerkraut and sausage do not. Other undesireable characters that can result from LAB include diacetyl (movie theater popcorn butter, rancid butter) and butyric acid (spoiled cheese, sweat).
Prevention of these LAB off-characters can typically be handled by inoculating for MLF with a cultured bacteria along with its nutritional supplement. Mitigation is difficult. Aeration can “blow-off” some of the offending aromas. Suppression is important to stop further production of the off-aroma. SO2 and limiting oxygen will stop the microbes so that the wine can be filtered and the off-aroma blended away. In the case of diacetyl, it is often prudent to wait, especially if MLF is ongoing. Formation of diacetyl is part of the MLF pathway. It may dissipate once MLF is complete.
Excessive sulfur dioxide (SO2) can impart a matchstick aroma to wine. High SO2 additions or the lowering of wine pH can result in excessive SO2 aroma. Aeration or oxygenation will reduce this character as the SO2 will bind with free oxygen and reduce the volatile portion of the component.